ABSTRACT
Introduction: Sovereign immunogens have been key in controlling COVID-19 in Cuba. The study of suboptimal response is a crucial step in reducing the risks of immunization strategies in the face of pandemics.
Objective: To characterize the suboptimal response of neutralizing anti-RBD antibodies and documented security profile.
Methods: A analytical observational and differentiated analysis study was carried out in 59 patients with suboptimal responses and immunized with the heterologous Soberana02/ SoberanaPlus scheme, vaccinated between March-May 2021 in Santiago de Cuba. A molecular virus neutralization test was used at a serum dilution of 1/100. A ˂70 % RBD-ACE2 binding inhibition response was managed as a suboptimal humoral response, while a ˂30 % response was managed as a critical humoral response.
Results: The arithmetic mean of the RBD-ACE2 binding inhibition response was 40.74 %. For a suboptimal humoral response, the lowest proportion of subjects was in the control group compared to the vaccinated group: 40 % vs. 22 % (p=0.000*). For a critical humoral response, the lowest proportion of subjects was in the vaccinated group 8 % vs. 30 % (p=0.000*). No serious adverse events were reported in vaccinated patients. Two types of adverse events were reported, with no definitive causal relationship and of mild intensity: pain at the injection site (46.6 %) and increased blood pressure (20 %).
Conclusions: The immunization scheme studied, under real-world conditions, was consistent with the results of clinical trials regarding the potent inhibitory antibody response, with an adequate safety profile.
Keywords: vaccines, anti-COVID vaccines, neutralizing antibodies, SARS-CoV-2.
RESUMEN
Introducción: Los inmunógenos Soberanos fueron clave en el control de la COVID-19 en Cuba. Un paso en la reducción de riesgos de las estrategias de inmunización ante pandemias.
Objetivo: Caracterizar la respuesta subóptima de anticuerpos anti-RBD neutralizantes y documentar el perfil de seguridad.
Método: Se empleó un estudio observacional analítico y análisis diferenciado en una serie de 59 sujetos con respuestas subóptimas del inmunizados con el esquema heterólogo Soberana02/Soberana Plus, entre marzo-mayo de 2021 en Santiago de Cuba. Se utilizó una prueba de neutralización molecular de virus a una dilución sérica de 1/100. Una respuesta de inhibición de unión de RBD-ACE2 ˂70 %, se manejó como respuesta humoral subóptima, mientras que una ˂30 % manejó como respuesta humoral critica.
Resultado: La media aritmética de la respuesta de inhibición de unión de RBD-ACE2 fue de 40,74%. Para una respuesta humoral subóptima la menor proporción de pacientes estuvo en el grupo de controles respecto de vacunados: 40% vs. 22% (p=0,000). Para una respuesta humoral critica la menor proporción de afectados estuvo en el grupo de vacunados 8 % vs. 30 %(p=0,000). No se reportaron eventos adversos graves en los pacientes vacunados. Se mostraron dos tipos de eventos adversos, sin relación de causalidad definitiva y de intensidad leve: dolor en el sitio de inyección (46.6 %) y aumento de cifras tensionales (20 %).
Conclusiones: El esquema de inmunización estudiado, fue consistente con los resultados de los ensayos clínicos respecto a la potente respuesta de anticuerpos inhibitorios, con un perfil de seguridad adecuado.
Palabras clave: vacunas, vacunas anti-COVID, anticuerpos neutralizantes, SARS-CoV-2.
Introduction
The immunogens of the Soberana line are based on the recombinant receptor binding domain (rRBD) fermented in higher mammalian cells at the Center for Molecular Immunology. With the obtaining of rRDB , and the extensive experience accumulated in the generation of vaccines, the Finlay Institute created its candidates Soberana 02 and SoberanaPlus , the first a macromolecular construct of 25 µg of rRBD conjugated with tetanus toxoid and the second, 25 µg rRBD dimeric adjuvanted with Aluminum Hydroxide gel. Its platform has demonstrated a potent neutralizing response in murine models, corroborated in clinical studies. 1, 2
The very design of protein subunit vaccines guarantees a broad safety profile, 3,4 despite requiring a greater number of doses to achieve an efficient adaptive immune response. Comparing a technologically and immunogenically superior vaccine platform in theory such as the Janssen vaccine, the 62 % efficacy of Soberana 02 in two doses acquires added value compared to it, despite the 66.3 % efficacy of the referred vaccine, 5 and without the risks of potentially fatal phenomena. 6
Despite its safety, the risk of obtaining poor responses is a critical variable to control in this vaccine platform. Its study and characterization are important for the design of robust and resilient strategies in the pandemic context.
An important indicator of the potential protective effect of a vaccine-induced immune response is the detection of neutralising antibodies. Neutralising antibodies are highly specific immunoglobulins with the ability to cut off the spread of a pathogen. The availability of these determinations is a crucial tool in the strategic planning applied to vaccines, with added value in pandemic contexts or when there is an increase in the circulation of pathogens that cause vaccine-preventable diseases.
This article presents the results of immunization with the heterologous two - dose Soberana02 regimen, with a third Soberana Plus dose in vaccinated subjects from the province of Santiago de Cuba, with the objective of characterizing the suboptimal response of neutralizing anti-RBD antibodies with the heterologous Soberana02/ Soberana Plus regimen.
Methods
Study design: Prospective longitudinal observational study developed during the implementation of the SOBERANA-INTERVENTION clinical study (study code IFV/COR/10; https://rpcec.sld.cu/ensayos/RPCEC00000360-Sp) in workers and collaborators of LABEX-CIM, in Santiago de Cuba.
Products under evaluation: SOBERANA 02 and SOBERANA Plus are injectable suspensions. Both are subunit vaccines based on the SARS-CoV-2 RBD, sequence Arg319-Phe541-(His )6 with a flexible C-terminal fragment including unpaired Cys538, produced in genetically modified CHO cells. In SOBERANA 02, 25 μg of RBD is conjugated to 20 μg of the tetanus toxoid (TT) carrier protein. In SOBERANA Plus-50 μg, the RBD is dimerized (d-RBD) through an interchain disulfide bridge Cys538-Cys538. Both vaccines use aluminum hydroxide as an adjuvant. SOBERANA 02 and SOBERANA Plus are produced under GMP conditions at the Finlay Vaccine Institute (IFV) and the Center for Molecular Immunology (CIM), in Havana, Cuba.
Molecular virus neutralization assay, based on antibody-mediated blockade of the RBD:hACE 2 interaction. This assay is an in vitro surrogate for the live virus neutralization assay. It uses recombinant RBD-mouse- Fc (RBD - Fcm ) and the host cell receptor hACE2-Fc (ACE2-Fch) as the coating antigen. Human antibodies against RBD can block the RBD- Fcm interaction with ACE2-Fch. The RBD- Fcm that was not inhibited can bind to ACE2-Fch, and is recognized by an alkaline phosphatase-conjugated anti- murine monoclonal antibody. This inhibition ELISA mimics the virus-host interaction at the molecular level. The inhibition ratio of the RBD:hACE 2 interaction at a serum dilution of 1/100 and the half-maximal virus molecular neutralization titer (mVNT50) were calculated; mVNT50 is the serum dilution that inhibits 50% of the RBD:hACE2 interaction. 7
For the purposes of this study, the results are referred to as suboptimal RBD-ACE2 binding inhibition response (SBR). A ˂70% RBD-ACE2 binding inhibition response was considered a suboptimal humoral response, while a ˂30% response was considered a critical humoral response.
Human Convalescent Serum Panel: A panel of convalescent serum samples (Cuban Convalescent Serum Panel, CCSP) was performed with sera from 68 patients recovered from COVID-19 (diagnosed by positive PCR) between March and November 2020, during the first epidemic peak in Cuba (13 with severe disease, 30 with mild disease and 25 asymptomatic). All patients gave their written consent to the National Center for Medical Genetics of Cuba in Havana, allowing the use of their samples for epidemiological research. In this study, the results of the inhibition of the RBD-hACE2 interaction (% of inhibition and molecular neutralization titer) were used with the same analytical method used for the vaccinated subjects. 8
Statistical analysis: Clinical data were retrieved from medical records and organized in a digital database using the services of the Microsoft Office Excel platform. For the statistical description, the arithmetic mean and median were used as summary measures of central tendency; the standard deviation and variance were used as measures of dispersion, as well as other coefficients of symmetry and linear correlation; for the summary of qualitative aspects, the results were expressed in percentages. For normality tests, QQ probability distribution graphs and the Jarque -Bera (JB) test were used. In the statistical analysis, graphic and computational means were used to determine the normality of the data distribution, and for the exploration of statistical significance, the Fischer test and Welch 's t test were used, assuming that α=0.05, as well as Hedges ' d for the estimation of the effect size.
Ethical considerations: The study will be governed by the general principles established in the documents adopted by the international community in relation to biomedical research on human subjects, established in the Declaration of Helsinki (update of the World Medical Assembly held in Brazil, 2013), 9 with the current state regulations according to the requirements of the national regulatory authority (Regulation 165/2000 of the CECMED), 10 as well as in the Good Clinical Practice Guide of the International Conference on Harmonization (ICH E6). 11
Safety profile: it was evaluated in all individuals with at least one dose of vaccination received, up to 28 days of follow-up. Face-to-face interrogation, physical examination and taking of vital signs (body temperature, measurement of respiratory rate, heart rate and blood pressure) were performed before each immunization and one hour later. A telephone consultation was made 30 days after the last immunization received.
Local AEs requested were pain, erythema, increased volume, induration and increased temperature at the site of administration. Systemic AEs requested were fever, malaise and rash.
The adverse events observed were classified according to preferred terms by organ system and intensity, according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE, Common Terminology Criteria for Adverse Events), which grades AEs as mild (grade 1): asymptomatic or mild symptoms, demanding only clinical observation, no treatment indicated; moderate (grade 2): requiring minimal, local or non-invasive treatment, event limiting activities of daily living (ADLs); severe (grade 3): clinically significant, but not immediately threatening, requires hospitalization or prolonged hospitalization, is disabling, limits ADLs to self-care; with life-threatening consequences (grade 4): demands urgent treatment and death (grade 5): related to AD.12
AE was also evaluated as severe, categorized as that which produces death, threatens life, produces permanent disability, significant disability or results in hospitalization or prolongation of hospitalization, linked to the vaccines evaluated; according to the outcome recovered, recovered with sequelae, persistent, resulting in death or unknown.
Results
59 vaccinated subjects, with a 1:1.7 ratio in favor of the female sex. 33.89 % had a history of chronic non-communicable disease, where hypertension was the most representative. The mean age was 44.22±2.43 years, with a standard deviation of 9.55 years. The mean in the female sex was 47.40±2.30 years (JB=1.5035; p=0.4715), with a standard deviation of 7.15 years. Regarding the male sex, the mean was 38.86±4.50 years (JB=1.5303; p=0.4652), with a standard deviation of 10.78 years.
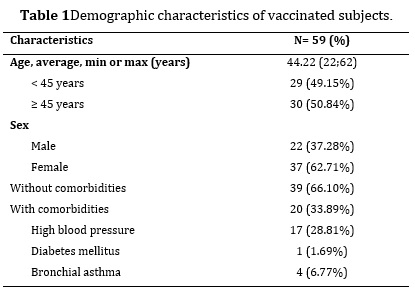
Between sexes, statistically significant differences with a moderate effect size were observed with respect to age (d=0.70; p=0.002). Likewise, between age groups, differences with a large effect size were observed (d=1.50; p=0.000*) and between subjects with and without health history, differences were observed with respect to age with a slight effect size (d=0.39; p=0.003).
In general, the mean inhibition of RBD-ACE2 binding is 75.73±5.83%, in an inhibition range of 11.3 and 95.2 %, being statistically significant in general that it is greater than 70 % inhibition (p=0.0293).
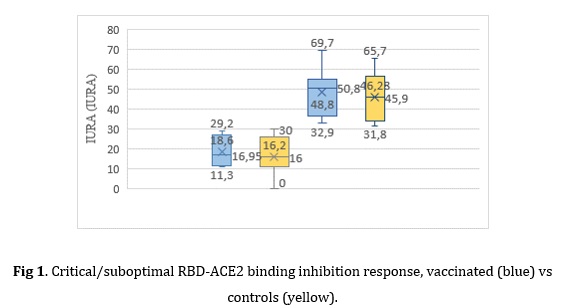
The analysis of the response medians, in general, confirms the presence of marked differences between the vaccinated subjects with respect to the controls in benefit of the immunized ones (75.73 % vs 48.24 %) with a large effect size (d=0.77, p=0.000*).
A lower proportion of subjects with suboptimal responses (IURA˂70 %) was observed in the control group compared to the vaccinated group: 40% vs. 22 % (p=0.000*); with no significant differences between inhibition means (p=0.1909). A lower proportion of subjects with critical responses (IURA˂30 %) was observed in the vaccinated group 8 % vs. 30 % (p=0.000*); with no significant differences between inhibition means (p=0.6468).The analysis of subjects with responses below 70 % did not show any marked differences in relation to age or sex, noting that 73.33 % of this set of subjects had an apparent health history. The arithmetic mean of the humoral response was 40.74 %. 80 % of them were over 45 years old, with responses >30 %<70 %, with a distribution by sex of 46.66 % women and 26.66 % men.
Fig 1. Critical/suboptimal RBD-ACE2 binding inhibition response, vaccinated (blue) vs controls (yellow).
No serious adverse events were reported in vaccinated patients. Only two types of adverse events were reported in this subgroup, with no definitive causal relationship and of mild intensity: pain at the injection site 46.6 % and increased blood pressure 20 %.
Discussion
This work reports for the first time data regarding the suboptimal response of the heterologous Soberana02+SuberanaPlus regimen in real-world subjects, compared to COVID-19 convalescents. Regardless of the sample size, representativeness is achieved with respect to the demographic characteristics of the starting population 13, 14 with working age, a group of interest in vaccination campaigns due to the high risk of contagion and its contribution to the transmissibility of SARS-CoV-2.
Subunit protein vaccines, despite presenting clear advantages in terms of manufacturing, storage and distribution, 15 have demonstrated an adequate clinical effect. 16 The use of a novel heterologous scheme distinguished the response of Cuban biotechnology to the confrontation of COVID-19. 17 Although the evidence is conclusive regarding the safety, immunization and generalization of the subunit protein vaccine platform, the risk of suboptimal responses is a variable to be controlled.
Serum antibody determinations, although not used to diagnose the presence or absence of current or previous SARS-CoV-2 infection, are particularly useful for assessing the presence of neutralizing antibodies, which act to prevent the virus from continuing to replicate, 18 which is an essential objective of anti-COVID vaccines in their aim to significantly impact the progression to severe forms of the disease. Correlates of protection based on these determinations have not been reported.
Antibody tests available for laboratory use are primarily enzyme-linked immunosorbent assay (ELISA) methods; these require relatively specialized equipment and biosafety procedures, 18 so their generalization is somewhat limited, although they do allow the creation of analytical capabilities in contexts with limited financial resources.
Based on observations made in the natural virus-host interaction, regarding a neutralizing antibody response that is predominantly directed to the RBD , 19 knowing that they are affected by the SARS-CoV- 2 variants, many clinical studies defined these antibodies as their primary endpoints of immunogenicity.
Few studies analyze in depth the data of subjects with responses that do not satisfy the response criteria defined as positive. In the studies carried out in Cuba with the Abdala vaccine, a response with a value greater than 30% was counted as a positive inhibition response. 20
In the Phase II study of the Abdala vaccine, the proportion of subjects with responses less than 30 % inhibition of 44.53 % was observed with the three-dose regimen of 25 μg , every 14 days, in contrast to the proportion observed in the 50 μg strength regimen which was 27.91 %, with a median inhibition of 55.5 % (95 % CI: 48 9--61 9) and 72.1 % (95 % CI: 65 9--77 7) respectively. 20 Compared to our results, the differences were statistically significant, in favor of the heterologous regimen compared to the three-dose regimen with 50 μg strength (27.91 % vs. 6.77 %, p=0.0006).
In the case of the clinical trial that explored the immunogenicity of the heterologous Soberana02/ SoberanaPlus regimen , the results were significantly superior, with a median inhibition of 85.5 % (95 % CI: 49.4 -- 93.1) with a proportion of subjects with responses less than 30 % of 12.73 %. 21 In comparison with our results, no significant differences are observed (p=0.3015).
Observations in this real-world subject group are consistent with results reported in rigorous clinical studies for the heterologous regimen, and superior results are evident compared to the other regimen. The application of a heterologous regimen with SoberanaPlus was established as a safe and effective strategy, with a lower proportion of subjects with suboptimal neutralizing antibody responses.
The main limitations of the study are related to the sample size of vaccinated persons and the sampling at 6 and 12 months for immunological studies, and larger national studies are needed. The availability of these data is crucial for designing more robust vaccination strategies.
The proportion of subjects with a suboptimal anti-RBD neutralizing antibody response is significantly lower in vaccinated subjects than in patients with the natural disease. No associated serious adverse events are reported. Therefore, it is concluded that under conditions of medical practice, the vaccination schedule studied induces a potent humoral response, with low levels of neutralising antibodies rarely seen, with an adequate safety profile.
Bibliographic References
- Valdes Balbin Y, Santana Mederos D, Paquet F, Fernandez S, et al. Molecular Aspects Concerning the Use of the SARS-CoV-2 Receptor Binding Domain as a Target for Preventive Vaccines. ACS Cent Sci. 2021[cited 20/01/2022];7(5):757-67. Available at : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8084267/
- Valdes Balbin Y, Santana Mederos D, Quintero L, Fernández S, Rodríguez L, Sánchez Ramírez B, et al. SARS-CoV-2 RBD-Tetanus Toxoid Conjugate Vaccine Induces a Strong Neutralizing Immunity in Preclinical Studies. ACS Chemical Biology. 2021[cited 20/01/2022];16(7):1223-33. Available at: https://hal.archives-ouvertes.fr/hal-03443835
- Arashkia A, Jalilvand S, Mohajel N, Afchangi A, Azadmanes A, Salehi Vasiri M, et al. Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects. Rev Med Virol. 2021[cited 20/01/2022];31(3):e 2183. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7646037/
- Tripathi NK, Shrivastava A. Recent Developments in Recombinant Protein-Based Dengue Vaccines. Front Immunol.2018.[cited 20/01/2022];9:1919 . Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6115509/pdf/fimmu-09-01919.pdf
- Centers for Disease Control and Prevention (CDCJ).Johnson & Johnson's Janssen COVID-19 vaccine & Johnson. [cited 20/01/2022]. Available at: https://stacks.cdc.gov/view/cdc/106729
- Centers for Disease Control and Prevention .Food and Drug Administration, FDA Maryland 2022 [updated April 13, 2021; accessed January 25, 2022]. Joint CDC-FDA Statement on Johnson & Johnson COVID-19 Vaccine. [cited 20/01/2022] Available at: https://stacks.cdc.gov/view/cdc/105057
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020[cited 20/01/2022];38 (9):1073-78. Available at : https://www.nature.com/articles/s41587-020-0631-z
- Chang Monteagudo A, Ochoa Azze R, Climent Ruiz Y, Macías Abraham C, Rodríguez Noda L, Valenzuela-Silva C, et al. A single dose of 100 ng / ml RNA in rats was compared with a placebo - controlled trial of 100 ng / ml RNA in rats . SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg Health Am. 2021. [cited 20/01/2022]; 4: 100079. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8442527/pdf/main.pdf
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 [cited 20/01/2022];310(20):2191-4. Available at: https://pubmed.ncbi.nlm.nih.gov/24141714/
- Cuba. Center for State Control of Medicines, Equipment and Medical Devices (CECMED). Regulation 165-2000: Good Clinical Practices in Cuba. 2016. [cited 20/01/2022] Available at: https://www.cecmed.cu/sites/default/files/adjuntos/Reglamentacion/Res_MINSAP-165-00.pdf
- The European Agency for the Evaluation of Medical Products. Guideline for good clinical practice. ICH Harmonized tripartite guideline . London: EMEA 2002. [cited 20/01/2022] Available at: https://ancei.es/wp-content/uploads/9/10/Normas-Buena-Practica-Clinica_EMA-2002.pdf
- NIH. National Cancer Institute (NCI).Bethesda,MD:NIH;2017. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. [cited 20/01/2022] Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Pérez Hernández HJ, Toledo Heredia M, Saurez Martínez G, García Céspedes RI. Safety and immunogenicity of the heterologous Soberana 02/Soberana Plus vaccination schedule in Santiago de Cuba. MEDISAN. 2022[cited 20/01/2022];26(3):[aprox. 15 p.]. Available at: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1029-30192022000300001&lng=es&nrm=iso&tlng=en
- Pérez Hernández HJ, Toledo Heredia M, Saurez Martínez G, García Céspedes RI. Safety and immunogenicity of the heterologous Soberana 02/Soberana Plus vaccination schedule in Santiago de Cuba. MEDISAN. 2022[cited 20/01/2022];26(3):[aprox. 15 p.]. Available at: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1029-30192022000300001&lng=es&nrm=iso&tlng=en
- Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021[cited 20/01/2022];6(1):104. Available at: https://www.nature.com/articles/s41541-021-00369-6
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020[cited 20/01/2022];586(7830):516-27. Available at: https://www.nature.com/articles/s41586-020-2798-3
- Chang Monteagudo A, Ochoa Azze R, Climent Ruiz Y, Macías Abraham C, Rodríguez Noda L, Valenzuela-Silva C, et al. A single dose of 100 ng / ml RNA in rats was compared with a placebo - controlled trial of 100 ng / ml RNA in rats . SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg Health Am. 2021. [cited 20/01/2022]; 4: 100079. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8442527/pdf/main.pdf
- Centers for Disease Control and Prevention (CDC). Interim Guidelines for COVID-19 Antibody Testing. [cited 20/01/2022] Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
- Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020[cited 20/01/2022];183(4):1024-42.e21. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7437498/
- Márquez L, Palenzuela DO, Figueredo M, Martín Y, Dueñas Carrera S, Valdés Y, et al. Immunogenicity and safety of the two-dose heterologous Ad5-nCoV / ZF2001 vaccine regimen against COVID-19 in adults in Cuba: a phase 3, randomised, observer-blind, placebo-controlled trial. Lancet Infect Dis. 2023[cited 20/01/2022];23(2):209-19. Available at: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00598-4/fulltext
- Toledo-Romaní ME, García-Carmenate M, Verdecia-Sánchez L, Pérez-Rodríguez S, Valenzuela-Silva C, Rodríguez-González M, et al. Safety and immunogenicity of anti-COVID-19 conjugated vaccine candidate SOBERANA 02: Phase IIb clinical trial in adults in Havana. MEDICC Rev. 2022[cited 20/01/2022];24(3-4):27-39. Available at: https://mediccreview.org/safety-and-immunogenicity-of-anti-covid-19-conjugated-vaccine-candidate-soberana-02-phase-iib-clinical-trial-in-adults-in-havana/
Conflict of interests
No conflicts of interest are declared.
Author contributions
Conceptualization: Héctor José Pérez Hernández
Data curation: Héctor José Pérez Hernández
Formal analysis: Héctor José Pérez Hernández, Giselle Saurez Martínez
Research: Héctor José Pérez Hernández, Giselle Saurez Martínez, Marlene Toledo Heredia, Rosa Iris García Céspedes
Methodology: Héctor José Pérez Hernández
Supervision: Héctor José Pérez Hernández
Validation: Héctor José Pérez Hernández, Giselle Saurez Martínez
Visualization: Héctor José Pérez Hernández, Giselle Saurez Martínez
Writing – original draft: Héctor José Pérez Hernández
Writing – review & editing: Héctor José Pérez Hernández, Giselle Saurez Martínez, Marlene Toledo Heredia, Rosa Iris García Céspedes
Percentage contribution:
Héctor José Pérez Hernández 50%
Giselle Saurez Martínez 20%
Marlene Toledo Heredia 15%
Rosa Iris García Céspedes 15%

